Novilux SMC™ Instrumentation
Ultrasensitive detection for any diagnostic setting


Proto 1 Exterior

Assay Processor

Optics & Fluidics
Phoenix Point-of-Care Platform
-
The Phoenix instrument is designed to operate in concert with a unique, low-cost consumable disc for fully automated processing.
-
Whole blood is processed to plasma via onboard centrifugation and incubated. The assay processor then adds buffers and moves analytes quickly though fluidic circuits to enable counting of single molecules from each analyte.
-
The consumable contains multiple fluidic circuits with each circuit handling one or two analytes. The system currently processes 4 analytes simultaneously with six and eight plex processing in development.
-
The "SAFE" QC system processes imagery and laser scans in real-time to ensure quality metrics are achieved.
-
Novilux designed and built key optical and fluidic components to optimize performance and reduce cost. By taking this approach, Novilux has achieved robust, high performance at low cost.
-
The result: The Phoenix platform packs a host of highly sophisticated and optimized elements into a tiny, low-cost, fully automated package generating Best in World performance at the Point-of-Care.
o Unmatched Sensitivity o Low-cost o Run time QC
Fast
Results in less than 9 minutes
Multiplexed
Optical & spatial multiplexing
Runtime QC
High integrity results every run
Rapid Development Platform
A wide range of content including quantitative and non-quantitative immunoassays and blood chemistries is suitable for the Phoenix PoC platform. Novilux designed the Rapid Development Platform (RDP) to facilitate development of SMC™ and non-SMC™ assays.
The RDP enables samples to be read in a micro-titer plate format after being processed in parallel on the bench using liquid or lyophilized reagents. This enables rapid prototyping using 1000’s of sample runs to generate a statistical understanding of new assay performance. The ability to develop assays in liquid format and then test lyophilized formulations of those same assays in parallel is key to rapid menu expansion of the platform.
By design, the multiplexed optics and signal processing engine in the RDP are identical to those in the Phoenix platform. The RDP is used for new assay development, incoming QC of buffers and reagents and confirmatory testing of samples and calibrators.
o Multiplexed high throughput assay and reagent development
o RDP enables rapid menu expansion for the Phoenix platform

Novilux Software Tools
The Point-of-Care and RDP platforms utilize the same software internally developed at Novilux. This is true for instrument control, runtime SAFE QC, results determination as well as the tools for post hoc data analysis.
Instrument Software

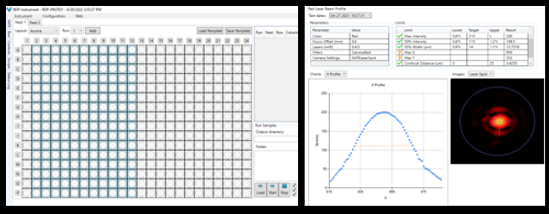
Point-of-Care Platform
(Status of instrument during run)
Rapid Development Platform
(384 well selection for analysis)
SAFE QC Interface
(Laser image in confocal stop)
Analysis Software

Signal processing interface
(SAFE QC Wash analysis)

Statistical Analysis
(LoBDQ, dynamic range, linearity...)
